N,N-DIMETHYL-P-NITROSOANILINE AND POTASSIUM LINOLEATE HYDROPEROXIDE AS SPIN TRAPS IN PROCESS OF HYDROXYL RADICALS FORMATION DURING CHLORIDE-FREE ELECTROLYSIS OF CONTAMINATED WATER
Сучасне матеріало- та товарознавство :: 1. Актуальнi питання наукового та практичного матерiалознавства
Page 1 of 1
 N,N-DIMETHYL-P-NITROSOANILINE AND POTASSIUM LINOLEATE HYDROPEROXIDE AS SPIN TRAPS IN PROCESS OF HYDROXYL RADICALS FORMATION DURING CHLORIDE-FREE ELECTROLYSIS OF CONTAMINATED WATER
N,N-DIMETHYL-P-NITROSOANILINE AND POTASSIUM LINOLEATE HYDROPEROXIDE AS SPIN TRAPS IN PROCESS OF HYDROXYL RADICALS FORMATION DURING CHLORIDE-FREE ELECTROLYSIS OF CONTAMINATED WATER
N.N. Barashkov
Micro-Tracers, Inc, San Francisco, CA
T.V. Sakhno
Poltava University of Economics and Trade, Ukraine
Irina Irgibaeva, Anuar Aldongarov, Artur Mantel
Eurasian National University, Astana, Kazakhstan
Micro-Tracers, Inc, San Francisco, CA
T.V. Sakhno
Poltava University of Economics and Trade, Ukraine
Irina Irgibaeva, Anuar Aldongarov, Artur Mantel
Eurasian National University, Astana, Kazakhstan
N,N-DIMETHYL-P-NITROSOANILINE AND POTASSIUM LINOLEATE HYDROPEROXIDE AS SPIN TRAPS IN PROCESS OF HYDROXYL RADICALS FORMATION DURING CHLORIDE-FREE ELECTROLYSIS OF CONTAMINATED WATER
Introduction. Chlorination is a well-established technology to disinfect water. It is presently more cost-effective than either UV or ozone disinfection. Chlorine disinfection is reliable and effective against a wide spectrum of pathogenic organisms. However, traditional chlorination has several disadvantages, including the following: a) the chloride residual, even at low concentration, is toxic to aquatic life; b) all forms of chlorine are highly corrosive and toxic, therefore, storage, shipping, and handling pose a risk, requiring increased safety regulations; c) chlorine oxidizes certain types of organic matter in wastewater, creating more hazardous compounds (e.g. trihalomethanes).
Various electrical processes have been proposed for disinfection and sterilization of contaminated waters and food. They have included ohmic heating via the passage of current at moderate voltage, high-voltage pulsing that disrupts and otherwise destroys bacteria, and electrolytic processes that produce oxidants at low voltage.
It is known that the mechanism of electrolytic disinfection depends on many parameters, including the nature of the electrolyte [1-3]. Relatively recently the mechanism of electrochemical (EC) wastewater disinfection has been discussed by Li et al. [4] who investigated the behavior of E.coli in solutions of NaCl, NaNO3 and Na2SO4. In comparison to direct chlorination, the EC process displayed a much stronger disinfecting capability than that of electrochlorination assumed for EC disinfection [5,6]. Study of the E.coli bacteria of wastewater treated by different means of disinfection suggested that the cells were likely killed during the EC treatment by chemical products with oxidizing and germicidal powers similar to that of ozone and much stronger that of chlorine.
In the present study water contaminated with E.coli and containing ammonium sulfate as electrolyte was subjected to electrolytic treatment in electrochemical cell with electrodes made of stainless steel at low alternating current (from 0.2 to 0.5 A) and initial voltage demand of 10-60 volts (close circulating system). The percentage of the initial concentration of bacteria which were destroyed by electrolysis was proportional to both treatment time and concentration of hydroxyl radicals. Detection of hydroxyl radicals formed during electrolysis was confirmed using two types of radical scavengers (spin traps): N,N-dimethyl-p-nitrosoaniline (RNO) and potassium linoleate hydroperoxide (PLH). The kinetic calculations for expenditure of hydroxyl radicals in case of both scavengers have been evaluated and conclusion that RNO is more efficient as spin trap compared to PLH has been made.
Experimental Part
Test water and bacterial counts
Deionized , DI, water i.e. top water purified with Ultra-Pure Water System, Model D8961, Barnstead/Thermolyne (Dubuque, IA) was used for the experiments before it has undergone any treatment. The preliminary bacterial load of the DI water was performed by using the Pour Plate Method for Heterotrophic Plate Count.
Procedure for electrochemical treatment
The experimental set up employed is shown on Fig.1. This apparatus includes the pump, 1, Flow meter, 2, transparent glass tube 3, which can be irradiated by visible light from the luminescent lamp, 4 (in this part of the study it will not applied), plastic electrochemical cell 5, with 10 electrodes made of stainless steel screen installed parallel to each other. The details of experimental electrochemical procedure have been published earlier [7,8].

Fig.1. Apparatus for electrochemical treatment includes the pump, 1, Flow meter, 2, transparent glass tube 3, which can be irradiated by visible light from the lamp, 4 (in this part of the study in will be not applied), plastic electrochemical cell, 5, with 10 electrodes made of round shape stainless steel screen.
Determination of hydroxyl radical formation has been evaluated by using N,N-dimethyl-p-nitrosoaniline (Sigma-Aldrich , Catalog # D172405) as well as potassium linoleate hydroperoxide (PLH), as spin traps [8]
Synthesis of PLH. Synthesis of potassium linoleate hydroperoxide (PLH) has been performed according to the published procedure [9] (privet, 1955) using linoleic acid (Sigma, Catalog # L1376) and lipooxidase enzyme prepared from Baker’s Nutri Soy Flour (Product of Protein Specialties Division, ADM Company, Decatur, IL). 150 g of Nutri Soy Flour was suspended in 1,000 ml. of acetate buffer at pH 4.5. The mix was stirred for 1 hr and filtered. The filtrate containing the enzyme was adjusted to pH 7 with 1 N potassium hydroxide and filtered again. Extracts prepared in this manner contained 50 units of lipoxidase activity per ml as measured by the published assay method [9]. The prepared enzyme extract was saturated with oxygen before mixing with 300 ml of solution prepared by neutralizing 5g of linoleic acid with 1N potassium hydroxide solution, following by addition of 1N ammonium hydroxide-ammonium chloride buffer at pH 9.0. The mixture was mixed vigorously by means of mechanical stirrer for 1 hr A peroxide number determined by iodometric titration [10] was about 600 meq/kg. The 300 ml of cold ethanol (0oC) was added to the mixture to stop the reaction. The entire mixture was then cooled to 0oC, acidified with 0.1N HCl to pH of 8.0 and extracted three times with 250 ml of cold ethyl ether. The prepared PLH has FTIR spectrum similar to spectrum reported in study [9].
RESULTS AND DISCUSSION
The dependence of the logarithm of number of living bacteria (Log n) in the treated deionized water containing E. Coli on treatment time (t) is depicted on Fig. 2. The first conclusion which can be made from these data is that the electrochemical treatment is much more effective sanitizing method than pumping the contaminated water through the screen electrodes without the voltage applied. The second conclusion is that there is a linear relation between Log n and t in case of the electrochemical treatment.
Parameters of k (a constant depending on the current density for a constant volume of treated water and a surface area of electrodes) and td (the time in which the straight lines intersect the time axis, i.e. Log n = 0) were evaluated in terms of equation (1), where the initial number of bacteria no, and current number of bacteria n, at time t is described as follows:
Log n = log no - k t (1)
The diagram in Fig. 2 enables one to calculate factor k and to determine such essential parameters of the disinfection process as the minimum time td required for complete elimination of bacteria. It corresponds to the intersection point of the experimental line with the time axis. In the same series of experiments, which are summarized Fig. 2, the factor k was estimated as 0.0635 min-1 and for Log no = 6.12, td was determined as 96.3 min.
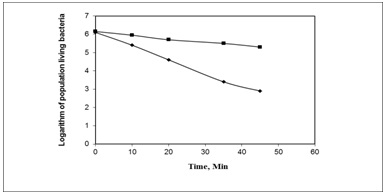
Fig.2. Live E. Coli count vs. disinfection time in ammonium sulfate solution with concentration 0.2% without electrolysis (■) and with electrolysis (
 ).
).Our experiments demonstrate that the percentage of the initial concentration of bacteria which were destroyed by electrolysis was proportional to both treatment time and concentration of hydroxyl radicals. Fig. 3 shows the chemical reactions between the spin traps and hydroxyl radicals
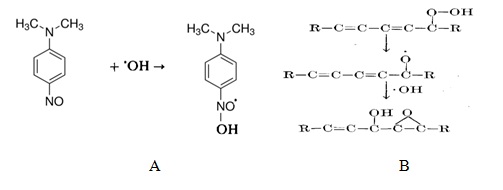
Fig.3 Interaction of N,N-dimethyl-p-nitrosoaniline (RNO) (A) and potassium linoleate hydroperoxide (PLH) with hydroxyl radicals [11,12].
According to data [11], interaction of RNO with OH-radiacals leads to formation much less reactive N-oxide radical (Fig. 3A). Reaction of PLH with OH-radicals is more complex [12]and possibly includes the formation of alkoxy radical and further radical combination with participation of hydroxyl radicals (Fig. 3B). The attractive feature of both reaction is significant changes in the absorbance spectra of RNO and PLH during their reaction with OH-radirals (Fig.4) which can be used for analytical control of hydroxyl radicals formation and consumption.

Fig 4. Absorbance spectra of RNO (A) and PLH (B) in water containing 0.2% of ammonium sulfate before pumping through the electrochemical cell (1) and after pumping through it for 10 min (2), 20 min (3), 40 min (4) and 60 min (5) under electrochemical conditions in case of presence of E.coli at similar starting concentration of bacteria.
Fig. 4 shows the change in the absorption spectra of RNO and PLH in ammonium sulfate aqueous solution contaminated with E.coli under the conditions of electrolysis. As we see, a solution flow through the electrolytic cell noticeably decreases the optical density at 440 nm for RNO and at 234 nm for PLH. In our previous study [8] we were able to show that in case of RNO solution the optical density decreases far more rapidly if electrolysis takes place in the bacteria free solution. Our recent experiments demonstrated that the spectral behavior of PLH solution in this respect is similar to the spectral behavior of RNO solution (Fig. 5).
It was assumed [8,13] that it was possible to neglect potential occurrence of other reactions involving hydroxyl radicals, excepting elimination of bacteria and interaction with RNO. In this case expenditure of OH radicals in electrochemical treatment can be described as follows:
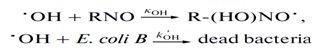
The kinetic model suggested for description of two competing reactions has been used at four different starting concentration of bacteria that allowed to implement the spectrophotometry of RNO for evaluation of reaction rate constant between OH-radicals and E.coli which had the value of 6.01 x 106 cfu/s [8].

Fig. 5. Absorbance of PLH in water containing 0.2% of ammonium sulfate before pumping through the electrochemical cell (1) and after pumping through it for 10 min (2), 20 min (3), 40 min (4) and 60 min (5) under electrochemical conditions (no E.coli is present).
In similar manner in this study we used the difference in the spectrophotometric behavior of PLH during electrolysis of its solution in absence of bacteria (Fig. 5) and in presence of E.coli (Fig. 4B). Four different starting concentrations of bacteria were used in these experiments (Table 1). The kinetic analysis was performed by using the same model for description of two competing reactions as it was proposed previously for RNO [8,13]:
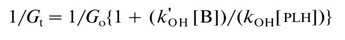 (2)
(2)where Gt is the rate of decreasing an absorbance value of PLH in the presence of bacteria, Go is the rate of decreasing an absorbance value of PLH in the absence of bacteria, [B] is the concentration of bacteria in solution, [PLH] is the PLH concentration in solution, and k’OH and kOH are the relevant reaction rate constants.
Table 1. Factor k and complete disinfection time td, calculated by equation (1) and E.coli elimination rate constant calculated by equation (2)

he DLH concentration was measured after 40 min treatment of 0.2% aqueous solution of ammonium sulfate. The 1/Gt vs. [B]/[DLH] is a linear relationship with the slope of 1/Go /kOH). The radical bacteria interaction rate constant was calculated on the assumption that DLH – OH-radical reaction rate constant is 1.2 1010 mol-1s-1, i.e. it is equal to this constant for RNO-OH-radical reaction.
The reason for found difference between the values of constant k’OH of 3.21 x 106 and 6.01 x 106 cfu/sec that are determined in case of using DLH and RNO, as spin traps, respectively, requires further investigation.
CONCLUSIONS
1) Chlorine free AC electrochemical disinfection with ammonium sulfate electrolyte is an effective method for disinfection of DI water contaminated with E.coli bacteria.
2)Use of RNO and DLH as spin traps experimentally confirmed formation of hydroxyl radicals in electrolysis of aqueous ammonium sulfate solutions. Hydroxyl radicals make an essential impact to the bactericidal process.
3) Under the same electrolytic conditions, the reaction rate constant describing the interaction between OH-radicals and bacteria determined in case of using DLH as spin trap are almost twice lower than this constant in case of using RNO. This observation requires further investigation.
REFERENCES
1. Y.Li, et al. , J.Food. Sci., 1994, v.59 (1), 23.
2. M.F.Slavik, et al. J.Food Protection, 1995, v.58 (4), 375.
3. Y.Li, et al., J.Food Protection, 1995, v.58 (12), 1330.
4. X.Y.Li, H.F.Diao, F.X.J.Fan, J.D.Gu, E.Env.Eng, 2004, v.130 (10), 1217.
5. T.Grahl, H.Markl, Appl. Microbiol. Biotechnology, 1996, v.45 (1/2), 148.
6. G.Patermarakis, E.Fountoukidis , Water Res., 1990, v. 24(12), 1491.
7. S.Yergeshbayeva, et al. “Chlorine-free electrochemical disinfection of water contaminated with E.coli”, Presented on 2013 Annual Meeting of American Institute of Chemical Engineers, San Francisco, 4-7 November, 2013.
8. N.N.Barashkov, et al. “Chlorine-free electrochemical disinfection of water contaminated with Salmonella typhimurium and E.coli B” , in Book “It’s All in the Water: Studies of Materials and Conditions in Fresh and Salt Water Bodies”, ACS Symposium Series, v.1086, Ed by M.A.Benvenuto, et all., Washington DC, 2011.
9. N.N.Barashkov, et al. Russian Journal of Electrochemistry, 2010, Vol. 46, No. 3, 306.
10.Nikolay N. Barashkov, David Eisenberg, Sylvan Eisenberg, Irina S. Irgibaeva, Gaukhar S. Shegebaeva and Tamara V. Sakhno Chlorine-free disinfection of water contaminated with Salmonella typhimurium by treatment with an alternating current: Role of hydrogen peroxide formation\\ 238th American Chemical Society National Meeting & Exposition August 16-20, 2009 Washington DC, USA http://envirofacs.org/Symposia/238_Washington/General.pdf
11.Nikolay Barashkov, David Eisenberg, Laila Lam, Irina Irgibaeva, . Tamara Sakhno, Tamara Novikova Chlorine-free disinfection of water contaminated with Campylobacter jejuni by treatment with an alternating current: Role of hydroxyl radicals formation 244th ACS National Meeting. Materials for Health & Medicine. 08/19/2012 - 08/23 /2012. Pennsylvania Philadelphia, PA.
12. Nikolay Barashkov, Tamara Sakhno, Irina Igribaeva, Sandugash Yergeshbayeva, Irina Zvonkina. Chlorine-free electrochemical disinfection of water contaminated with Escherichia coli: Role of electrode materials // 248th ACS National Meeting & Exposition August 10-14, 2014 San Francisco, CA •
13.Nikolay Barashkov, Tamara Sakhno, Irina Irgibayeva Chlorine-free disinfection of water contaminated with E.coli by combination of electrolysis and photochemical treatment: Role of electrode material 250th American Chemical Society National Meeting & Exposition Innovation from discovery to application 462 - ENVR: Division of Environmental Chemistry August 16 - 20, 2015 Boston, MA United States https://ep70.eventpilotadmin.com/web/page.php?page=IntHtml&project=ACS15fall&id=2232533
14.Nikolay Barashkov, Tamara Sakhno,Irina Irgibaeva 428 - Fenton reaction as a step of chlorine-free electrochemical disinfection of water contaminated with E. coli: Role of hydroxyl radicals and singlet oxygen formation // 251st American Chemical Society National Meeting & Exposition San Diego, CaliforniaUnited States Dates: March 13-17, 2016 https://ep70.eventpilotadmin.com/web/page.php?page=IntHtml&project=ACS16spring&id=2371937
Last edited by Admin on Thu Mar 15, 2018 10:25 am; edited 1 time in total
 Similar topics
Similar topics» INFLUENCE OF OPTICAL VIBRATIONS ON ENERGY ACTIVATION OF THE CHARGE CARRIER TRAPS AND THE THERMOLUMINESCENCE OF SILICON ORGANIC POLYMER
» DEVELOPMENT OF ADVANCED TECHNOLOGY OF CHLORINE-FREE WASTEWATER DISINFECTION
» DEVELOPMENT OF ADVANCED TECHNOLOGY OF CHLORINE-FREE WASTEWATER DISINFECTION
Сучасне матеріало- та товарознавство :: 1. Актуальнi питання наукового та практичного матерiалознавства
Page 1 of 1
Permissions in this forum:
You cannot reply to topics in this forum